Mechanisms of pathogenicity
In animal mycoplasmas, mechanisms of pathogenicity and determinants of virulence are still largely unknown. However, attachment of mycoplasmas on host cell surfaces is an essential step in tissue colonization and subsequent disease pathogenesis and is therefore considered to be a major virulence factor. Adhesion of animal mycoplasmas to host cells is a multifactorial event involving major and accessory adhesins as well as receptor components of the host. Some animal mycoplasma species exhibit flask-like cell morphology with a protruding tip structure that serves as an attachment organelle whereas adhesins of animal mycoplasmas lacking a tip structure may be distributed on their surface. Only for few animal mycoplasmas the molecular features of major adhesins have been identified and characterized (see mycoplasma profiles), while adhesins of most animal mycoplasmas are still largely unknown.
Although mycoplasmas were thought to be exclusively extracellular bacteria that remain attached to the surface of epithelial cells, intracellular localization has been demonstrated to occur in several mycoplasma species (Fig. 1). The capability to enter a protecting niche may provide these organisms with a unique opportunity for resisting host defense and antibiotic treatment and may contribute to the chronicity of infections. Invasion of host cells may also provide mycoplasmas with the ability to spread within a host by crossing mucosal barriers and gaining access to internal tissues.
Fig. 1. Intracellular localization of Mycoplasma cynos strain 2297 in HeLa cells using double immunofluorescence confocal microscopy. Note, intracellular localization (red) and extracellular localization (yellow) of mycoplasma cells. Credits: Joachim Spergser (Vetmeduni Vienna)
Several mycoplasmas have shown to produce biofilms and microcolonies, which may enable their survival not only in the environment but also within the host, perhaps contributing to chronic infections and reducing their susceptibility to antimicrobial treatment.
Some mycoplasmas produce capsular polysaccharides which might have a direct toxic effect on host cells, but certainly play a role in membrane integrity, adhesion to host cells and resistance to phagocytosis.
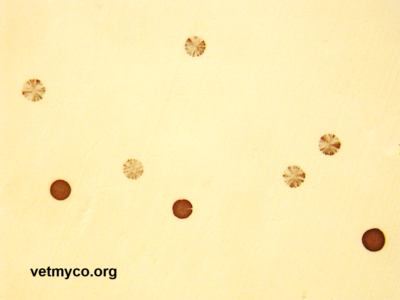
The discovery of sophisticated genetic systems that enable mycoplasmas to rapidly alter the antigenic make-up on their membrane surface providing phenotypic plasticity has been one of the major developments in mycoplasma research (Fig. 2). Phenotypic plasticity is achieved by ‘antigenic variation’ referring to the capacity of an organism to alternate between phenotypes in a reversible manner, encompassing phase variation (ON/OFF expression of a particular antigen) and antigenic variation (expression of alternative forms of a particular antigen including size variation and domain shuffling). Both cases can contribute to epitope masking in which the epitopes are subject to variable surface exposure due to a secondary protein that blocks accessibility. Since most of the variable surface components of mycoplasmas are lipoproteins that are highly immunogenic, phase and antigenic variation has thought to be a powerful mechanism for subversion or evasion of adaptive immune responses resulting in chronic or recurrent infections. Phase and antigenic variation have also been proposed to generate variants with altered colonization abilities providing mycoplasmas the flexibility to reach and adapt to different niches within the host. However, in many cases, the exact role that variable proteins may play in biological functions remains speculative.
Fig. 2. Variation in expression of surface antigens of mycoplasmas revealed by colony immunoblotting. Positive and sectored immunostaining of Mycoplasma agalactiae colonies reflecting the alternate expression state of a Vpma (variable protein of Mycoplasma agalactiae). Credits: Rohini Chopra-Dewasthaly (Vetmeduni Vienna)
The limited biosynthesis capabilities of mycoplasmas entail dependence on host microenvironments to supply biochemical precursors required for the biosynthesis of macromolecules. Competition for these biosynthetic precursors by mycoplasmas may disrupt host cell integrity and may alter host cell function. Furthermore, mycoplasmas are forced to scavenge for high-energy compounds and metabolites in host infection processes and uptake is facilitated by active transporter systems (e.g. ATP-binding cassette (ABC) transporter).
Release of toxic metabolites such as hydrogen peroxide and superoxide radicals by adhering mycoplasmas have been incriminated to cause oxidative damage to host cell membranes. Hydrogen peroxide production may accompany the metabolism of sugars or certain organic acids to acetate and carbon dioxide. During this process NAD+ is reduced to NADH and eventually regenerated via NADH oxidase (Nox) activity. This oxidation requires molecular oxygen which is reduced to hydrogen peroxide or water. An alternate release of hydrogen peroxide involves the metabolism of glycerol. Thereby, a glycerol kinase phosphorylates the imported glycerol in glycerol-3-phosphate which is oxidized into dihydroxyacetone phosphate (DHAP) and hydrogen peroxide via L-α-glycerol-3-phosphatase oxidase (GlpO) activity.
Furthermore, release of ammonia during arginine or urea metabolizing processes may result in cytotoxic effects.
Certain mycoplasmas (e.g. ureaplasmas) have shown to produce IgA proteases which may facilitate colonization by degrading this important component of the mucosal immune system. Most recently, activity of the Mycoplasma Ig binding-Mycoplasma Ig protease (MIB-MIP) system in host-pathogen interactions has been described in infected animals.
Altogether, host cell damage and resulting clinical manifestations appear to be mainly due to pathologic host immune reactions and inflammatory responses rather than to direct toxic effects of mycoplasma components. The complex network of interaction between mycoplasmas and the host immune system involves mycoplasma-induced specific and nonspecific immune reactions. Beside protection, specific host immune responses have also shown to play a role in the development of lesions and exacerbation of mycoplasma-induced diseases. Mycoplasmas may exert a wide range of nonspecific immunomodulatory effects upon cells of the immune system by (i) inducing suppression or stimulation of B and T lymphocytes, (ii) inducing cytokines and chemokines, (iii) increasing or decreasing the cytotoxicity of macrophages, natural killer cells, and T lymphocytes, (iv) enhancing the expression of cell receptors, (v) activating the complement cascade, (vi) inducing autoimmunity, (vii) modulating programmed cell death (apoptosis), and (viii) perturbation of tolerance to self-antigens.
References
- Citti, C. and R. Rosengarten. 1997. Mycoplasma genetic variation and its implication for pathogenesis. Wien. Klin. Wochenschr. 14-15: 562–568.
- Citti, C., Nouvel, L.X and E. Baranowski. 2010. Phase and antigenic variation in mycoplasmas. Future Microbiol. 5: 1073–1085.
- Citti, C. and A. Blanchard. 2013. Mycoplasmas and their host: emerging and re-emerging minimal pathogens. Trends Microbiol. 21: 196–203.
- Razin, S., Yogev, D. and Y. Naot.1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62: 1094–1156.
- Rocha, E.P. and A. Blanchard. 2002. Genomic repeats, genome plasticity and the dynamics of Mycoplasma evolution. Nucleic Acids Res. 30: 2031–2042.
- Rosengarten, R., Citti, C., Glew, M., Lischewski, A., Droesse, M., Much, P., Winner, F., Brank, M. and J. Spergser. 2000. Host-pathogen interactions in mycoplasma pathogenesis: Virulence and survival strategies of minimalist prokaryotes. Int. J. Med. Microbiol. 290: 15–25.
- Rosengarten, R., Citti, C., Much, P., Spergser, J., Droesse, M. and M. Hewicker-Trautwein. 2001. The changing image of mycoplasmas: from innocent bystanders to emerging and reemerging pathogens in human and animal diseases. In: Mühldorfer, I. and K.P. Schäfer (eds.), Emerging Bacterial Pathogens, Contrib. Microbiol. Vol. 8, S. Karger, Switzerland.
- Rottem, S. 2003. Interaction of mycoplasmas with host cells. Physiol. Rev. 83: 417–432.
- Sirand-Pugnet, P., Citti, C., Barre, A. and A. Blanchard. 2007. Evolution of Mollicutes: down a bumpy road with twists and turns. Res. Microbiol. 158: 754–766.
- Waites, K.B., Katz, B. and R.L. Schelonka. 2005. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin. Microbiol. Rev. 18: 757–789.
- Zimmerman, C.-U. 2014. Current insights into phase and antigenic variation in mycoplasmas. In: Browning, G. and C. Citti (eds.). Mollicutes: Molecular biology and pathogenesis. Caister Academic Press, UK.